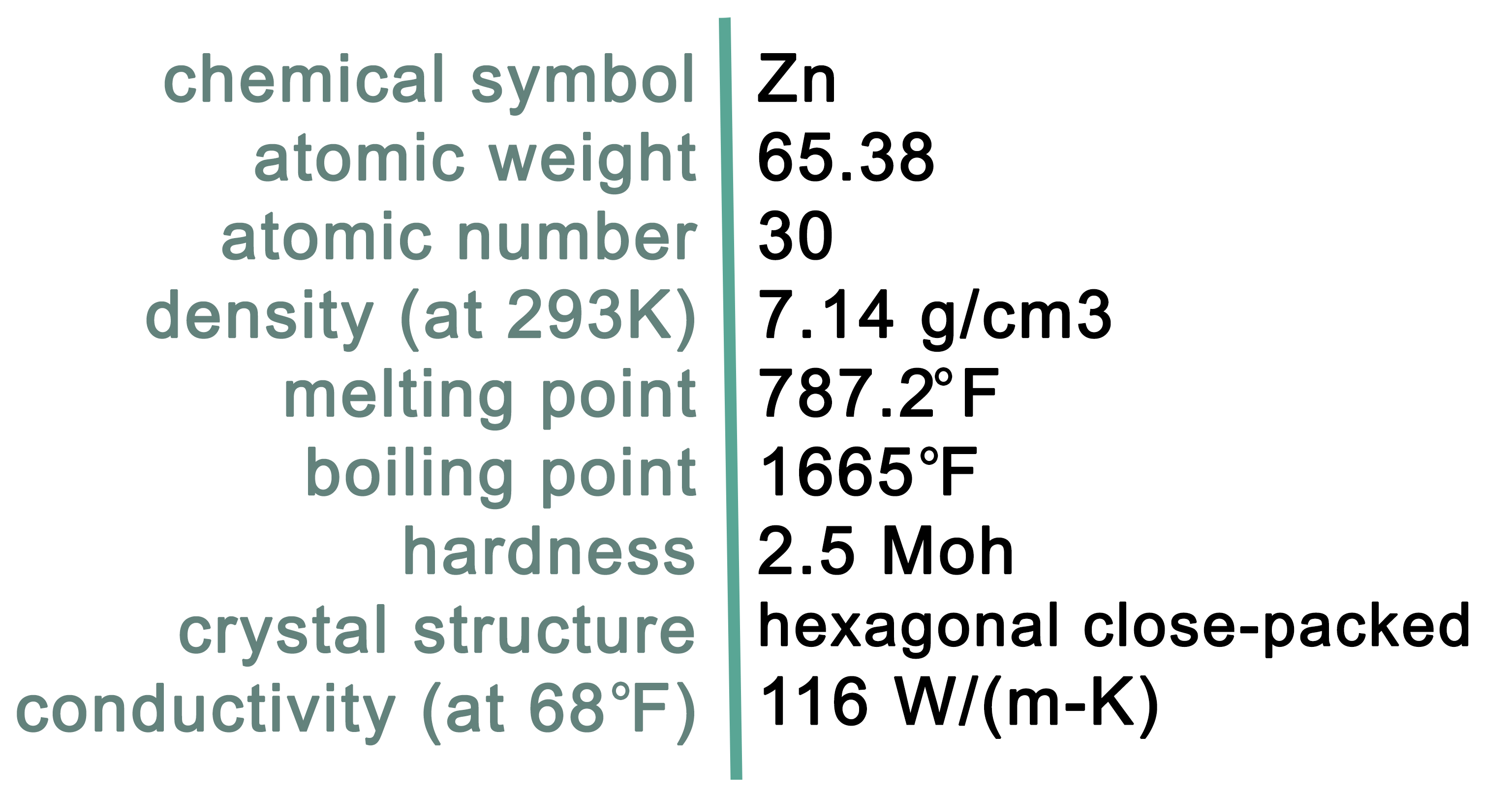
Characteristics of Zinc
Zinc is a bluish-white, lustrous metal with the chemical symbol Zn. It is the 24th most abundant element in Earth's crust and the 4th most widely consumed metal globally, behind iron, copper, and aluminum. At room temperature, zinc is a slightly brittle metal, but it becomes malleable at 100° C (212° F). Zinc and other base metals, such as copper and lead, are commonly found together in mineral deposits. Some of the important zinc-enriched minerals reported in Virginia are listed in Table 1.

| Mineral Name | Chemical Formula | Specific Gravity | Al % |
|---|---|---|---|
| Sphalerite | (Zn,Fe)S | 3.9-4.1 gm/cc | 67.1 |
| Smithsonite | ZnCO3 | 4.4-4.5 gm/cc | 52.15 |
| Hemimorphite | Zn4(Si2O7)(OH)2•H2O | 3.4-3.5 gm/cc | 58.16 |
| Wurtzite | (Zn,Fe)S | 3.9-4.1 gm/cc | 67.1 |
| Hydrozincite | Zn5(CO3)2(OH)6 | 3.5-4.0 gm/cc | 59.56 |
Table 1: Minerals containing the element Zinc

Components of a horizontal axis wind turbine coated by galvannealed steel
Uses of Zinc
Zinc is considered a "critical mineral" in domestic metallurgy. The element serves as an essential component in galvanized steel, which is currently in high demand for wind turbines, solar power systems, and electric vehicles (EV). Zinc oxides are used in a wide variety of medicines and nutritional supplements and in the manufacture of rubber in a chemical process known as vulcanization. About three-fourths of zinc consumed in the U.S. is used as metal, mainly as a coating to protect iron and steel from corrosion (galvanized metal), as alloying metal to make bronze and brass, as zinc-based die casting alloy, and as rolled zinc. A thin layer of zinc, just 0.02 mm thick, can make steel last for up to a century and sometimes more before it needs maintenance. The remaining one-fourth of zinc in the U.S. is consumed as zinc compounds, mainly by the rubber, chemical, paint, and agricultural industries. With a standard electrode potential (SEP) of 0.76 V, zinc is used as an anode material for batteries. Zinc is also an essential nutritional element for humans, animals, and plants; it is the second most common trace metal, after iron, naturally found in the human body. The value of zinc mined in 2021, based on zinc contained in concentrate, was about $2.4 billion (U.S. Geological Survey Mineral Commodity Summaries, 2022).

Sphalerite from the Austinville-Ivanhoe Mining district, Wythe County, Virginia (Photo courtesy: James St. John)
Zinc Geology
In the Earth's crust, zinc abundance averages about 75 parts per million (ppm), or about 0.0075%. Its main ore mineral is sphalerite, which is a zinc sulfide mineral with the chemical formula ZnS. Sphalerite is found in a variety of mineral deposit types, including sedimentary exhalative, Mississippi-Valley Type (MVT), and volcanogenic massive sulfide deposits. Many minable deposits of sphalerite are formed when the hydrothermal activity or contact metamorphism brings hot, acidic, zinc-bearing fluids in contact with carbonate rocks. There, sphalerite can be deposited in veins, fractures, and cavities or in replacement deposits. In these deposits, sphalerite is frequently associated with galena, dolomite, calcite, chalcopyrite, pyrite (and other sulfides), marcasite, and pyrrhotite. All natural sphalerite contains concentrations of various impurities, which generally substitute for zinc in the cation position in the lattice. The most common cation impurities are cadmium, mercury, and manganese, but gallium, germanium and indium may also be present in relatively high concentrations (hundreds to thousands of ppm). Other source minerals for zinc include smithsonite (zinc carbonate), hemimorphite (zinc silicate), wurtzite (another zinc sulfide), and sometimes hydrozincite (basic zinc carbonate). With the exception of wurtzite, all these other minerals are formed by weathering of the primary zinc sulfides.
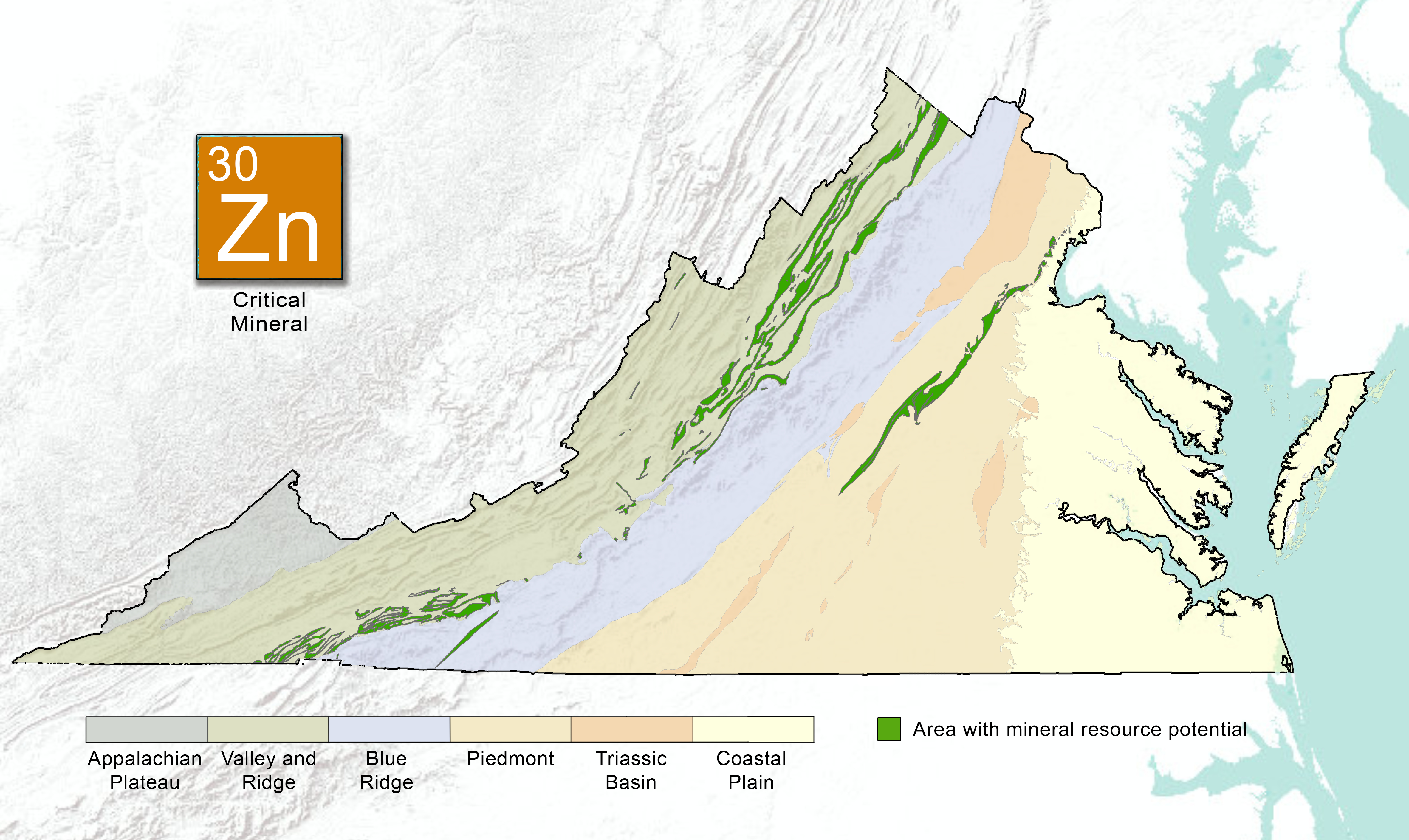
In Virginia, zinc source minerals have been identified in most of the geological provinces (Dietrich, 1990). Sphalerite has been reported from nearly all the counties west of the Blue Ridge Province and from a number of counties in the Piedmont Province. Smithsonite and hemimorphite occur in trace amounts at all Virginia zinc deposits (Dietrich, 1990; Table 1).
| Mineral System | Deposit Type | Geologic Provinces |
|---|---|---|
| Basin Brine Path | Mississippi-Valley Type (MVT) | Valley and Ridge |
| Volcanogenic Seafloor | Polymetallic sulfide | Piedmont |
| IOA-IOCG | Polymetallic sulfide S-R-V (Skarn-Replacement-Vein) | Blue Ridge |
| Mafic magmatic | Central Atlantic Magmatic Province (CAMP) | Mesozoic Basins |
| Porphyry Sn (granite-related) | Greisen (Sn, W, Be, Zinc) | Piedmont and Blue Ridge |
Table 2: Prospective zinc mineral systems, deposit types (Hofstra and Kreiner, 2020), and geologic provinces in Virginia
Zinc in Industry
Worldwide, 95% of zinc is mined from sulfidic ore deposits, with major mining locations in China, Australia, and Peru. Global zinc resources are reported to be about 1.9 billion tons (U.S. Geological Survey, Mineral Commodity Summaries, 2022). In 2021, zinc was mined in the U.S. in five states, including Idaho, Missouri, New York, Washington, and Tennessee. In Virginia, historic zinc mines, prospects, and occurrences are known in the Valley and Ridge, Blue Ridge, Piedmont, and Mesozoic Basins Provinces. Iron-copper-zinc and lead-zinc-iron-barite, commonly occur in closely parallel zones, overlap, or extend for miles along strike with locally different metal combinations. All these deposits appear to be originally formed from warm metal-rich saline brines or submarine hot springs that flowed along fractures or mega-fractures associated with rift systems or island arcs
Valley and Ridge ProvinceZinc occurs in the Valley and Ridge Province as strata-bound Mississippi-Valley Type (MVT) deposits within the Shady Dolomite of Cambrian age, and the Beekmantown Formation of Ordovician age. Most deposits within the Shady Dolomite and Rome Formation are located in Wythe and Smyth Counties. A few are located in Pulaski and Montgomery Counties. The most important deposits of zinc-lead ores within the Shady Dolomite are located in the Austinville-Ivanhoe mineral district in Wythe County. The overall zinc production from the ore district is estimated to be over 1.3 million short tons from the 1870s to 1981 (Sweet and others, 1989). In 2020, Virginia Energy, in cooperation with the USGS National Geological and Geophysical Data Preservation Program (NGGDPP), concluded a preservation effort to archive historic mining records from The New Jersey Zinc Company in Austinville, Virgnia. The deposits within the Beekmantown Formation are located in Augusta, Rockingham, and Shenandoah counties. The most important mine within the Beekmantown Formation is the Bowers-Campbell Mine, Rockingham County (Sweet and others, 1989).
Blue Ridge ProvinceZinc occurs in two main deposit types in the Blue Ridge Province, massive sulfide and vein-hosted deposits. Zinc was mined from strata-bound and syngeneic massive sulfide zinc- and copper-bearing pyrrhotite deposits in the Gossan Lead district of Carrol, Grayson, and Floyd Counties. It is estimated that there is 2.0% zinc in the sulfide bodies in the Gossan Lead. This deposit is enclosed in the clastic metasedimentary rocks of the Ashe Formation of the Lynchburg Group (Precambrian age). Small amounts of zinc, lead, and copper minerals are found in veins and greisens associated with granitoid plutons in the Irish Creek-Yankee Horse Ridge-Buck Mountain areas of Rockbridge and Amherst counties.
Piedmont ProvinceIn the Piedmont Province, zinc has been mined from localized, massive sulfide lenses in the Chopawamsic Formation of Cambrian age and the Ta River metamorphic suite. The Chopawamsic Formation crops out for 110 miles northeast to southwest across a region in central Virginia covering Louisa to Buckingham counties. The metals occur as highly localized simple sulfide enrichments in quartz veins, as strata-bound deposits in fractures, and as strata-bound disseminations (Sweet and others, 1989). Highly localized simple sulfide enrichments with minor amounts of lead occur in pyrite massive sulfide lenses.
Copper, lead, and zinc are also found within the intermediate and felsic metavolcanic rocks of Chopawamsic Formation and the gold-pyrite belt of north-central Virginia. These sulfide bodies may contain more than 10 million tons of pyrite, copper, lead, and zinc in the Mineral District, Louisa County (Pavlides and others, 1982). In the Andersonville District in Buckingham County, exploration drilling identified a cluster of sulfide-mineralized zones associated with ferruginous quartzites in the Chopawamsic Formation. Two of these zones contained 4.68% and 4.87% of Zn, with significant potential for future exploration (Young, 1981; Sweet and others, 2016).
Mesozoic BasinsHistorical lead-zinc mining occurred in the Mesozoic-age Culpeper Basin located in the northern Piedmont province, mainly in Orange and Prince William counties.Zinc occurrence in the Culpepper Basin may associate with 1) contact metasomatic aureoles near the basaltic feeder dikes and sills, 2) strata-bound deposits derived from migrating metal-enriched connate brines and precipitation with organic matter, and 3) syngenetic accumulations related to basin sedimentation during the Mesozoic (Sweet and others, 2016).
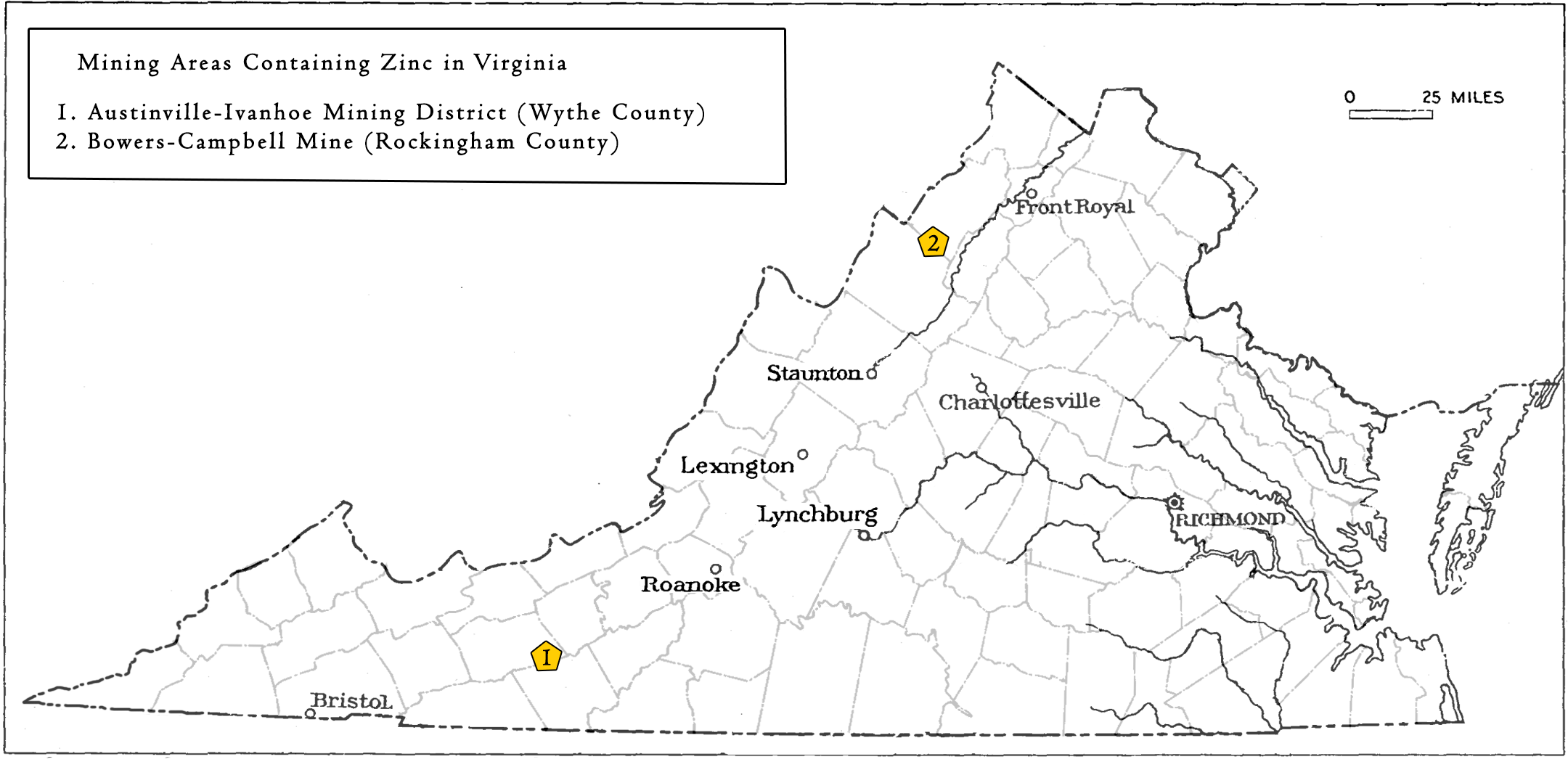
Significant zinc mining areas in the Valley and Ridge
Selected References:
Dietrich, R.V., 1990, https://www.energy.virginia.gov/commerce/ProductDetails.aspx?productID=1293. Department of Mines, Minerals, and Energy, Division of Mineral Resources, Charlottesville, VA.
Foley, N. K., and Craig, J. R., 1989, Mineralogy and geochemistry of the lead-zinc ores of the Austinville-Ivanhoe District, Wythe County, Virginia: Virginia Division of Mineral Resources, Publication 88, p. 23-39
Luttrell, G. W., 1966, Base- and precious-metal and related ore deposits of Virginia: Virginia Division of Mineral Resources, Mineral Resources Report 7, 167 p.
Sweet, P. C., Good, R. S., Lovett, J. A., Campbell, E. V. M., Wilkes, G. P., and Meyers, L. L., 1989, Copper, lead, and zinc resources in Virginia: Virginia Division of Mineral Resources, Publication 93, 185 p.
Sweet, P.C., Lassetter, W.L., and Sherwood, W.C., 2016, Non-Fuel Mineral Resources in Virginia, in, The Geology of Virginia. Virginia Museum of Natural History, Special Publication 18.
Watson, T. L., 1907, Mineral Resources of Virginia: Lynchburg, Virginia, Jamestown Exposition Commission, 618 p. (available as Virginia Division of Mineral Resources, 2003, Digital reprint of T. L. Watson's 1907 Mineral resources of Virginia: Virginia Division of Mineral Resources Publication 175, [CD-ROM; 2003, September 1].
Weinberg, E. L., 1980, Austinville mining - 1756 to present: Virginia Division of Mineral Resources, Virginia Minerals, v. 26, n.1, p.11.

.jpg)
