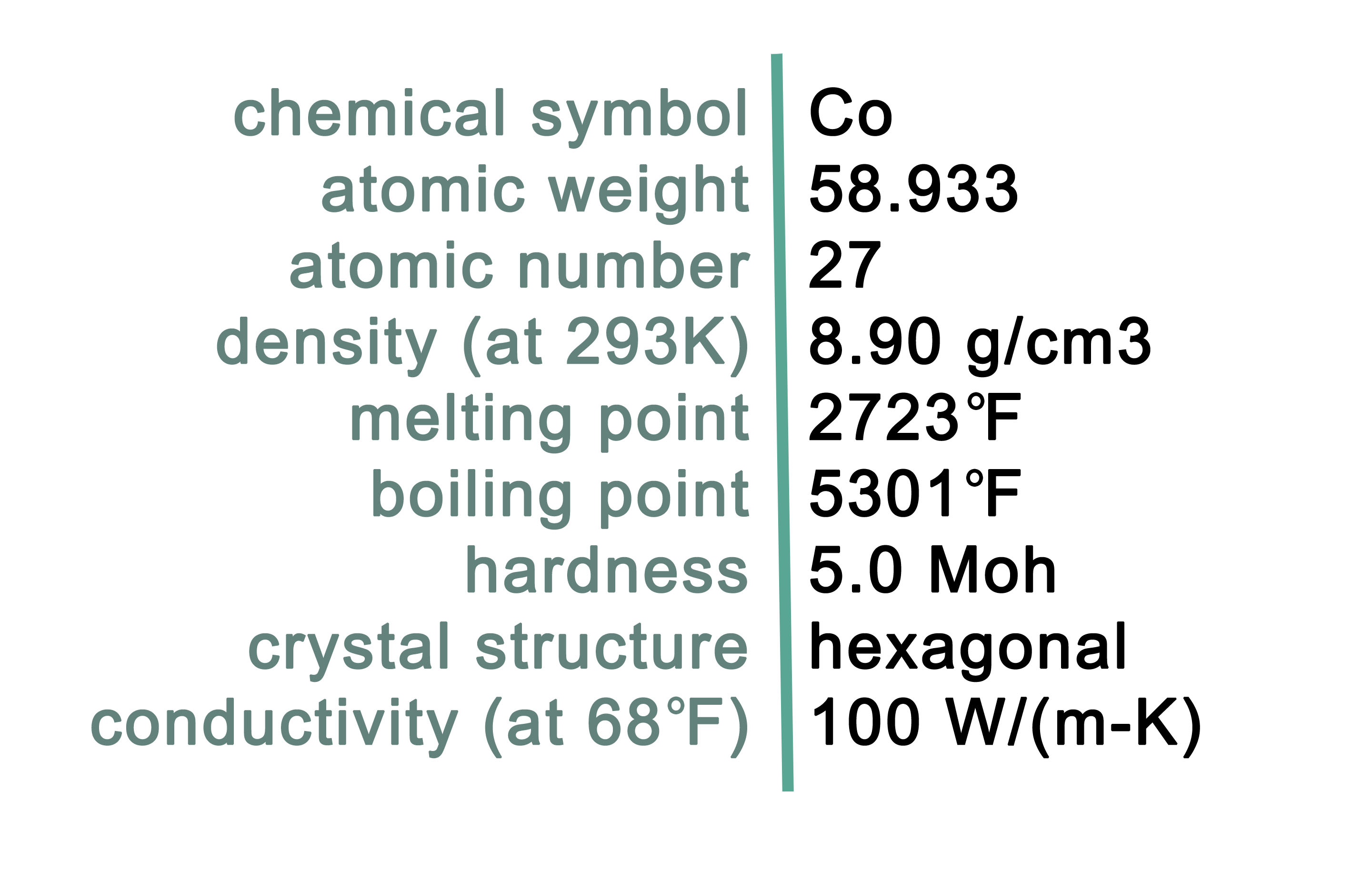
Characteristics of Cobalt
Cobalt is a grayish-white metal with the chemical symbol Co. As a pure metal, cobalt is hard and brittle. It makes a strong alloy with other metals, has a high melting point and low conductivity. Cobalt is predominantly used for producing brilliant blue pigments. Pure cobalt does not exist on Earth, rather cobalt is always bound with other elements as compounds which are common across the globe. Cobalt is also an essential nutrient for animal health. Cobalt deposits are commonly in the form of the minerals listed in Table 1.

| Mineral Name | Chemical Formula | Specific Gravity | Co % |
|---|---|---|---|
| Cattierite | CoS2 | 4.82 gm/cc | 47.89 |
| Safflorite | CoAS2 | 7.48 gm/cc | 21.25 |
| Glaucodot | CoAsS | 6.22 gm/cc | 26.76 |
| Stutterudite | CoAs3 | 5.95 gm/cc | 17.95 |
| Cobaltite | CoAsS | 6.35 gm/cc | 35.52 |
| Carrollite | CuCo2S4 | 4.83 gm/cc | 28.56 |
| Pentlandite | (Ni,Fe,Co)9S8 | 5.27 gm/cc | 67.40 |
| Linnaeite | CoCo2S4 | 4.88 gm/cc | 57.95 |
| Siegenite | Co3S4 | 4.85 gm/cc | 14.51 |
| Erythrite | Co3(AsO4)2 8(H2O) | 3.12 gm/cc | 29.53 |
Table 1: Minerals containing the element Cobalt

Modern electric cars, such as the Tesla Roadster,
require cobalt for their rechargeable batteries.
Uses of Cobalt
Cobalt has been used by humans for thousands of years. In ancient times, it was used to add a brilliant blue color to ceramics, jewelry, and even glass. In modern times, cobalt is considered a "critical mineral" in domestic metallurgical applications that serve aerospace, defense, energy, electronics, telecommunications, and transportation technologies (Fortier and others, 2018). Cobalt remains an important strong blue colorant, but is primarily used within rechargeable batteries for electronics and electric vehicles. It also is an important commodity for aircraft engines as a strong metal superalloy. Cobalt has a variety of other applications in the industrial, commercial, and military sectors. Airbags require cobalt during their production, cobalt is used in ink and varnishes as a drying agent, and in magnetic alloys. Cobalt composites can be made into wear-resistant diamond or cutting tools for mining, drilling, construction, and metalworking. When combined with steel, cobalt adds significant strength and workability.

A cubic cobaltite crystal from Canada.
Photo courtesy of Robert Lavinsky, irocks.com
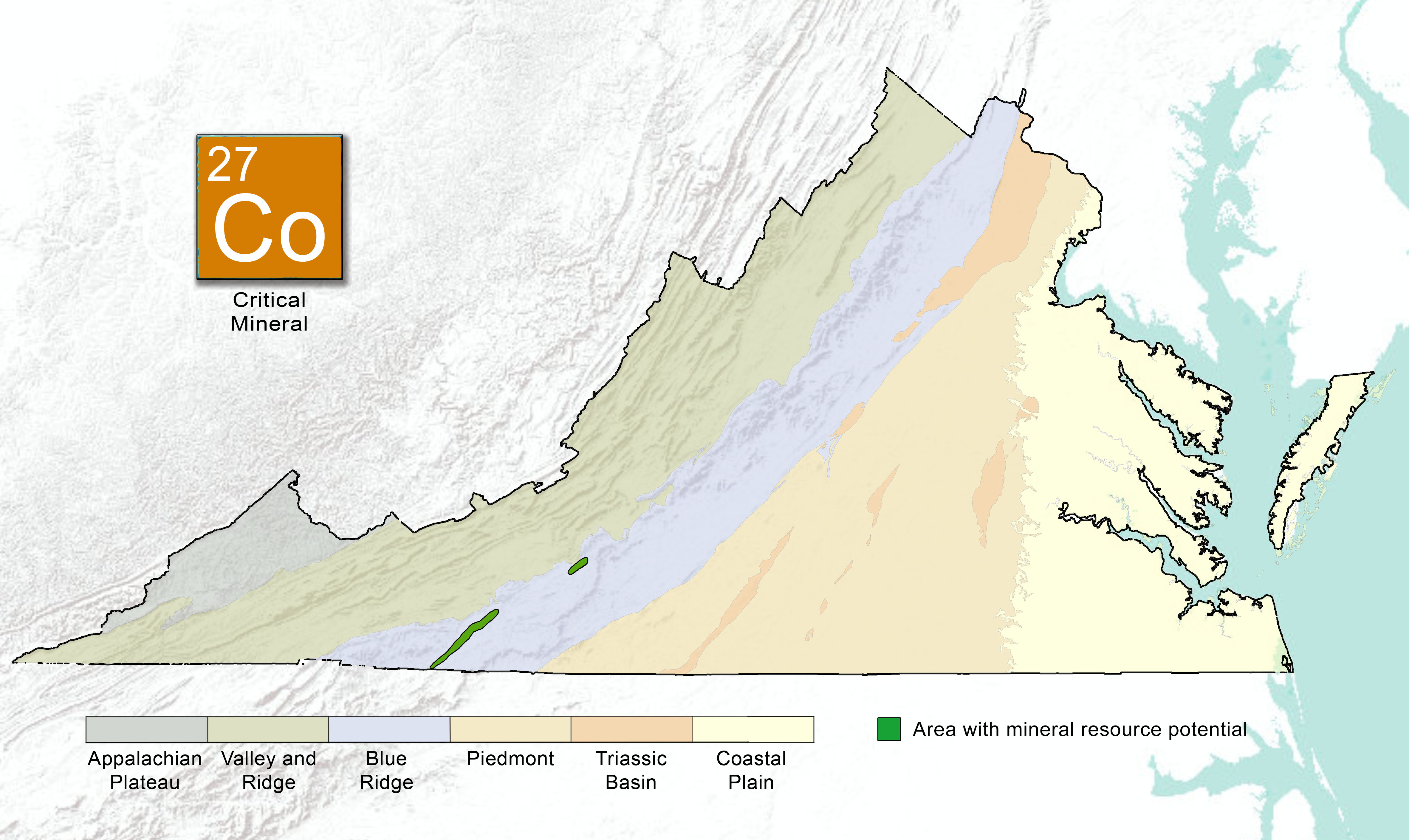
Cobalt Geology
Cobalt is common in the Earth's crust, bound with other elements to form a variety of minerals. This element is found in highest concentrations when incorporated into minerals (pentlandite and linnaeite) as magma or lava cools to form igneous rocks such as dunite, serpentinite, and basalt. These are known as magmatic Nickel-Cobalt sulfide deposits. Cobalt can also be found as stratiform sediment-hosted Copper-Cobalt deposits in siliciclastic or carbonate rocks. A third example of a cobalt deposit is a cobalt-rich laterite deposit that may form as ultramafic rock weathers into regolith rich in nickel and cobalt. Like manganese, cobalt can be found in relatively high concentrations (but not easily obtainable) on the sea floor as precipitated nodules and crusts.
| Mineral System | Deposit Type | Geologic Provinces |
|---|---|---|
| Chemical Weathering | Supergene Cobalt | Blue Ridge, Valley and Ridge |
| Basin brine bath | Sediment hosted or replacement Cu-Co | Valley and Ridge |
| Marine Chemocline | Fe-Mn | Valley and Ridge |
| Subduction and Rift Magmatic Provinces | Iron Oxide Copper Gold, Polymetallic S-R-V | Blue Ridge |
| Mafic Magmatic | Sulfide | Blue Ridge |
| Volcanogenic Hydrothermal / Metamorphic | SEDEX massive sulfide modified by regional structure and metamorphism | Blue Ridge |
Table 2: Prospective cobalt mineral systems, deposit types (Hofstra and Kreiner, 2020), and geologic provinces in Virginia
Cobalt in Industry
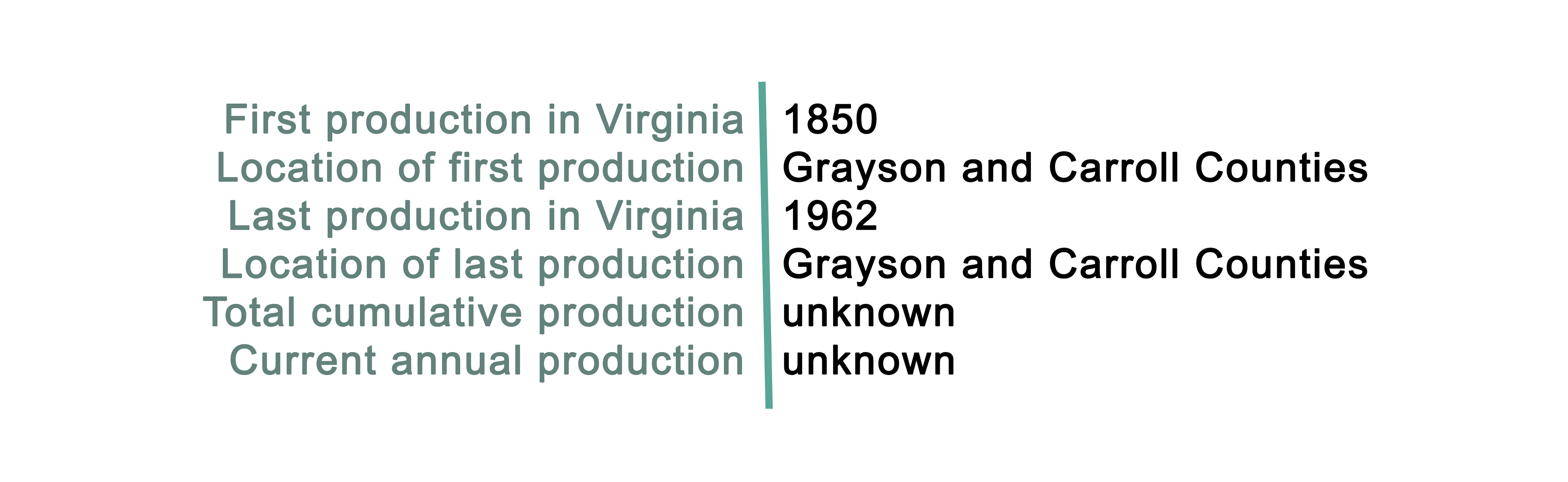
Pure cobalt does not occur on Earth, although cobalt-bearing minerals are common globally. Cobalt is often mined as a byproduct of nickel and copper. Cobalt is largely mined in Kinshasa (the Congo), but also in several other countries including China, Russia, and Canada. The U.S. maintains a supply of cobalt in the National Defense Stockpile and is 72 percent import reliant from several countries including Norway, Japan, China, and Canada. Within the United States, cobalt is mined mainly as a byproduct in Missouri and Michigan. Although cobalt is an essential nutrient for life, at high concentrations it becomes toxic. Concerns related to cobalt mining include the release and concentration of cobalt into the air, soil, and streams surrounding mine and refinery sites.
In Virginia, cobalt has historically been identified as a secondary or tertiary commodity at many mine sites in the Blue Ridge Province. The cobalt discovered in the Commonwealth is associated with other sulfide or metallic elements such as iron, copper, arsenic, and nickel. These sulfide ores can be found in veins, or irregularly distributed throughout the metamorphosed volcanic or magmatic rocks.
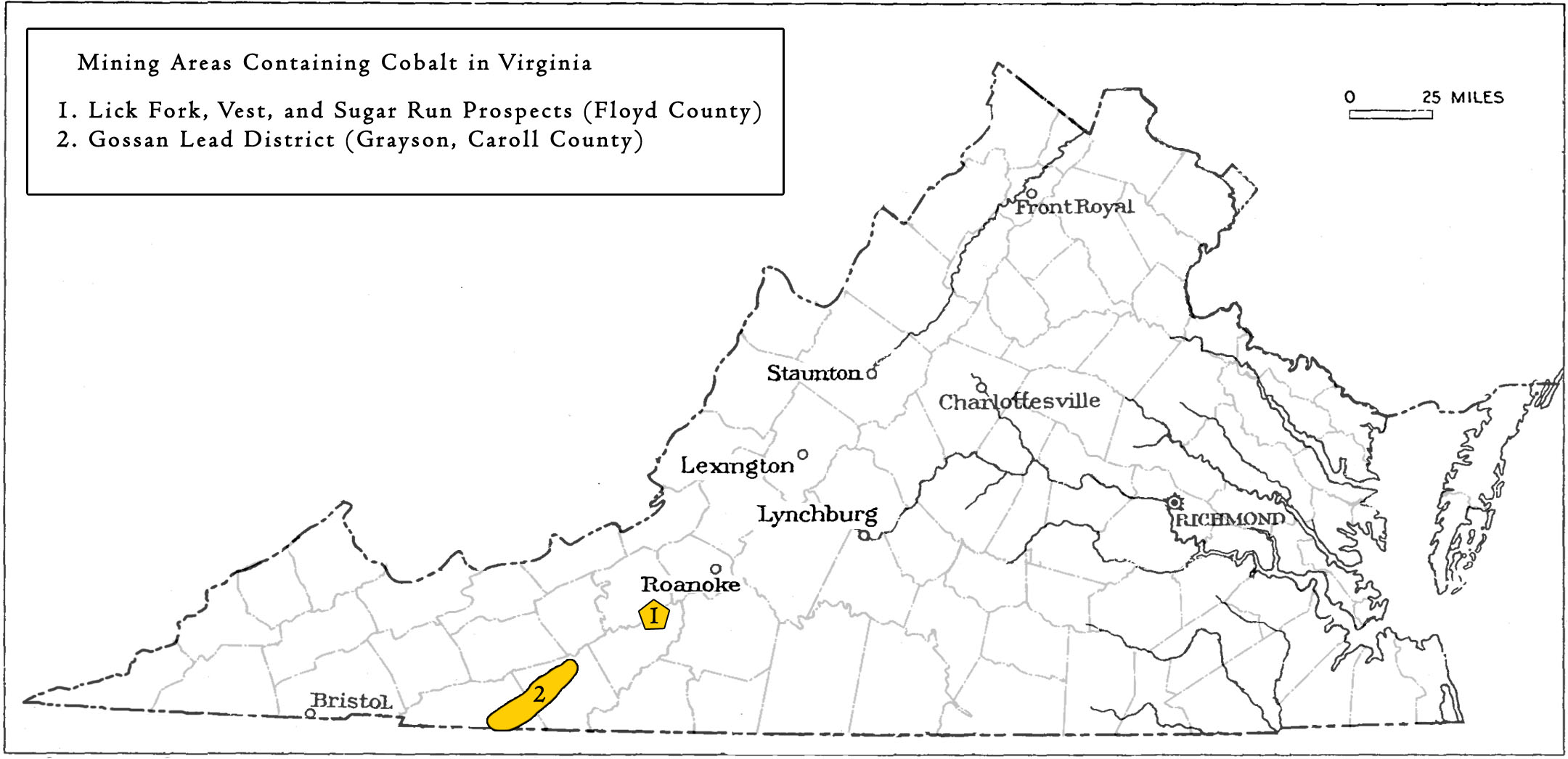
Mining locations containing Cobalt in Virginia.

Dump from Vest Prospect next to a stream.
Modified
from Sweet and Bell, 1980.
Occurrences of iron, nickel, copper, and cobalt mineralized rock exist in northern Floyd County (Watson, 1907). Here, three prospects were dug in the early 20th century. Although some of these locations were prospected intermittently for decades, none produced cobalt on an economic scale.
The Lick Fork prospect (also known as Mackusick, Flat Run, and John Light's Mine) was built in 1904, although sulfide-related ore had been identified in this location prior to the Civil War (Ross, 1935). Here, the sulfide ores are hosted by mafic and ultramafic rocks ranging in composition from pyroxene syenite to gabbro (Craig and Higgins, 1975). The ore bearing gabbro was estimated by Watson (1907) to be about 18-22 feet wide in the main adit. These rocks intrude medium-grained granulite-facies metamorphic rocks on the north side of the Fries fault zone. The Lick prospect was expanded from 1904 to 1907 by the Virginia Nickel Corporation, which opened several exploratory shafts and adits exposing disseminated and semi-massive sulfide zones. The minerals pyrrhotite, pentlandite, pyrite, chalcopyrite, ilmenite, magnetite, and violarite (a secondary oxidation mineral of pentlandite) were identified. Samples of violarite are reported to contain up to 9.7 weight percent cobalt (Craig and Higgins, 1975).
At the Vest prospect, located about two miles to the northeast of Lick Fork, similar mafic intrusive rocks have undergone metamorphism resulting in chlorite-tremolite schist within biotite gneiss (Walsh-Stovall and others, 1989). The mafic intrusive rocks originally hosted a suite of sulfide minerals, now well oxidized, that is similar to that found at Lick Fork. Exploratory work in 1924 included a 15 foot vertical shaft and a 75 foot-long adit (Sweet and Bell, 1980; Grosh, 1949). Four exploratory holes were drilled in 1936, and two more were added by the U.S. Bureau of Mines in 1944 (Walsh-Stovall and others, 1989). Samples revealed sparsely disseminated sulfide zones measuring up to 5 feet in thickness and containing 0.28 percent cobalt (Grosh, 1949). There are no records of mineral production from either Lick Creek or Vest (Sweet and Bell, 1908; Sweet and others, 2016).
A third cobalt prospect, named Sugar Run, was dug in 1902. Approximately three miles northeast of Vest prospect, Sugar Run consisted of small underground workings, including one shaft and a 40 foot adit (Dietrich, 1959). Similar to Lick Fork and Vest prospects, the primary commodity at Sugar Run was nickel in pentlandite, although this location did not produce economically.
Carroll-Grayson Counties
Cobalt has been identified within the Gossan Lead Sulfide District. The district stretches from the North Carolina border north across both Grayson and Carroll Counties, and partially into Wythe and Pulaski Counties (Stose and Stose, 1957). The sulfide deposits of interest are strata-bound massive and semi-massive and occurring in a narrow northeast-trending seventeen mile-long belt. The structurally-controlled mineralized zone contains en echelon veins that generally parallel the foliation of the metasedimentary host rocks, which include quartz- mica gneiss and schists with intercalated amphibolite units of the Neoproterozoic Ashe Formation (Lynchburg gneiss) (Stose and Stose, 1957). The sulfide ores consist primarily of pyrrhotite with lesser amounts of pyrite, chalcopyrite, sphalerite, and galena, but other economic minerals in the district include barite, magnetite, rutile, ilmenite, staurolite, spessartine, and kyanite (Stose and Stose, 1957).
The host rocks in the Gossan Lead are interpreted to be deposited as marine turbidite or flysch sequences with syngenetic sulfide deposits that were modified by structural deformation and metamorphic recrystallization (Gair and Slack, 1984; Kinkel, 1962; Magee, 1968). All of the rocks in the district have been folded and broken by thrust faulting, and these faults and related shears appear to have served as important controls on the circulation of hydrothermal fluids and ore deposition (Stose and Stose, 1957). Gossan and limonite that formed at the weathered surface of the sulfide ores can be traced for more than 60 miles extending into Floyd County to the northeast.
The Gossan Lead District was mined primarily for copper beginning as early as 1789. Mining for production occurred by 1850 following the discovery of similar deposits in southeastern Tennessee in 1843 (Sweet and others, 1989; Magee, 1968). The Toncray mine in Floyd County was opened initially on a pyrrhotite vein that also contained chalcopyrite and large amounts of the supergene mineral chalcocite (Watson, 1907). This was followed by mine developments at Betty Baker, Copperas Hill, and in the area of lron Ridge. In 1854 there were eight operating mines in the Gossan Lead district, and during the first half of 1855 these mines reportedly produced about 700 thousand tons of ore containing chalcocite that averaged 25 percent copper (Watson, 1907). As the rich secondary copper ores were depleted, attention was turned to the gossan zones that were mined for iron starting in about 1880. The brown iron ore was smelted locally in several nearby charcoal forges. Beginning in 1905, the pyrrhotite-rich sulfide ores at Iron Ridge were mined to produce sulphuric acid. Underground mining operations commenced in the mid-1930s and continued until the mines were closed in 1962 (Craig and others, 1971). Henry and others (1979) reported the average grade of sulfide deposits in the Gossan Lead to be about 0.5 weight percent copper, 0.1 weight percent lead, and 2.0 weight percent zinc. Gair and Slack (1984) estimated the total production plus reserves in the district to a depth of 984 feet to be about 18 thousand tons. Based on an evaluation of mineral dressing options for typical ores to a depth of 1640 feet in the Betty Baker and Lineberry mine areas, Corriveau (1956) had earlier estimated reserves (including gangue) in the Gossan Lead district to be up to 181 thousand tons.
Selected References:
Corriveau, M.P., 1956, Mineral dressing studies on the Great Gossan Lead ore from Carroll County,Virginia: Bulletin of the Virginia Polytechnic Institute Engineering Experiment Station Series No. 113, v. 49, n. 11, 79 p.
Craig, J.R., Sears, C.E., Gilbert, M.C., and Hewitt, D.A., 1971, The Gossan Lead district: Geological Society of America Southeastern Section Annual Meeting - Guidebook to Appalachian tectonics and sulfide mineralization of southwestern Virginia, Blacksburg, VA, May 5-9, field trip guidebook no, 5, p. 25-38.
Craig, J.R., and Higgins, J.B., 1975, Cobalt- and Iron-Rich Violarites from Virginia. American Mineralogist, Volume 60.
Corriveau, M.P., 1956, Mineral Dressing Studies on the Great Gossan Lead Ore from Carroll County, Virginia: Bulletin of the Virginia Polytechnic Institute Engineering Experiment Station Series, No. 113, v.49, n. 11.
Dietrich, R.V., 1959, Geology and Mineral Resources of Floyd County of the Blue Ridge Upland,Southwestern Virginia. Bulletin of the Virginia Polytechnic Institute Engineering Experiment Station Series,No. 134.
Fortier, S.M., Nassar, N.T., Lederer, G.W., Brainard, J., Gambogi, J., and McCullough, E.A., 2018, Draft Critical Mineral List - Summary of Methodology and Background Information - U.S. Geological Survey Technical Input Document in Response to Secretarial Order No. 3359: U.S. Geological Survey Open-File Report 2018-1021, 15 p.
Gair, J. E., 1978, Massive sulfides of Virginia field trip guidebook; Geol. Correlation Programme Project no.60: Correlation of Caledonian stratabound sulfides, 112 p.
Gair, J.E., and Slack, J.F., 1984, Deformation, Geochemistry, and Origin of Massive Sulfide Deposits, Gossan Lead District, Virginia: Economic Geology v. 79.
Grosh, W.A., 1949, Investigation of Vest nickel prospect, Floyd County, Virginia: U.S. Bureau of Mines Report of Investigations R.I. 4491, 4 p.
Henry, D.K., Craig, J.R., and Gilbert, M.C., 1979, Ore mineralogy of the Great Gossan Lead, Virginia:Economic Geology v. 74, p. 645-656.
Kline, M. H. and Ballard, J., 1949, Investigation of the Great Gossan Lead, Carroll County, Virginia: U.S.Bureau of Mines Rept. Inv.4532,39 p
Hofstra, A.H., and Kreiner, D.C., 2020, Systems-Deposits-Commodities-Critical Minerals Table for the Earth Mapping Resources Initiative: U.S. Geological Survey Open-File Report 2020-1042.
Luttrell, G.W., 1966, Base- and Precious-metal and Related Ore Deposits of Virginia. Virginia Division of Mineral Resources, Mineral Resources Report 7.
Magee, M., 1968, Geology and Ore Deposits of the Ducktown District, Tennessee, in Ridge, J.D. [ed], Ore Deposits of the United States, 1933-1967: American Institute of Mining, Metallurgical, and Petroleum Engineers, Graton-Sales volume I.
Poole, J. L., 1973, Iron sulfides mines in Virginia: Virginia Minerals, vol.19, no.3, p.29-33.
Ross, C.S., 1935, Origin of the Copper Deposits of the Ducktown type in the Southern Appalachian Region, U.S. Geological Survey, Professional Paper 179.
Stose, A.J., and Stose, G.W., 1957, Geology and mineral resources of the Gossan Lead District and adjacent areas in Virginia: Virginia Division of Mineral Resources Bulletin 72, 233 p.
Sweet, P.C., Good, R.S., Lovett, J.A., Campbell, E.V.M., Wilkes, G.P., and Meyers, L.L., 1989, Copper, lead, and zinc resources in Virginia: Virginia Division of Mineral Resources Publication 93, 185 p.
Sweet, P.C., Lassetter, W.L., and Sherwood, W.C., 2016, Non-Fuel Mineral Resources in Virginia, in The Geology of Virginia. Virginia Museum of Natural History, Special Publication 18.
Walsh-Stovall, C., Robinson, E.S, Rimstidt, J.D., and Stovall, R.L., 1989, Exploration for Magmatic Sulfide Deposits in the Virginia Blue Ridge, in Contributions to Virginia Geology - VI, Virginia Division of Mineral Resources, Publication 88.
Watson, T.L., 1907, Mineral Resources of Virginia, Lynchburg, VA, J.P. Bell Company, 618 p.
Wrightson, W., Jr., and Misra, K.C., 1982, Hydrothermal origin of nickel sulfide mineralization in the Lick Fork ultramafic-mafic intrusion, Virginia [abs]: Geological Society of America Abstracts with Programs combined NE/SE sectional meeting, Washington, DC, March 25-27, 1982, v. 14, n. 1-2, p. 97.
Young, R.S., 1956, Sulfides in Virginia: Division of Mineral Resources Virginia Minerals v. 2, No. 1, p. 1-7.
Young, R.S., 1981, Sulfide zones, in Geologic investigations in the Willis Mountain and Anderson quadrangles, Virginia: Virginia Division of Mineral Resources Publication 29, p. 17-47
.jpg)
